Toward Visible-Light Photochemical CO2‑to-CH4 Conversion in Aqueous Solutions Using Sensitized Molecular Catalysis/Cyclic voltammetry in various conditions
From ChemWiki
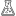 investigation
investigation
| analyte | anl conc [mM] | E0/0 [eV] | E0/0 type | E1/2 [eV] | *Ered [eV] | *Eox [eV] | *Redox type | Ered1 [eV] | Ered2 [eV] | Ered3 [eV] | Eox1 [eV] | Eox2 [eV] | Eox3 [eV] | solvent | solvent vol [ml] | electrolyte | electrolyte purity | el conc [M] | int ref comp | scan rate [mV/s] | scan number | potential window | scan dir | gas | T [°C] | condition [nm] | WE | WE SA [mm²] | CE | RE | Details | include | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | -2, -1.4; | 0.1 |
|
100 | argon | glassy carbon | 7.07 | platinum | SCE | Yes | |||||||||||||||||||||||
| 0.5 | -2, -1.4; | 0.1 |
|
100 | CO | dark | glassy carbon | 7.07 | platinum | SCE | Yes | ||||||||||||||||||||||
| 0.5 | -2, -1.4; | 0.1 |
|
100 | CO | irr. >420, 60 min | glassy carbon | 7.07 | platinum | SCE | Yes | ||||||||||||||||||||||
| 0.5 | -2, -1.4; | 0.1 |
|
100 | CO | TEA (0.05 mM), irr. >420 nm, 1 min | glassy carbon | 7.07 | platinum | SCE | Yes | ||||||||||||||||||||||
| 0.5 | ; | 0.1 |
|
100 | CO | TEA (0.05 mM), irr. >420 nm, 60 min | glassy carbon | 7.07 | platinum | SCE | Yes |
The results show an increase in current when the solution is irradiated and triethylamine (TEA) is added (see condition column).

