Rhenium(I) trinuclear rings as highly efficient redox photosensitizers for photocatalytic CO2 reduction
 publication
publication
| About |
|---|
|
A molecule on this page has a mistake in its structure or is missing a part. Please edit the structure or redraw a new molecule using the replace molecule workflow.
|
|
This page about a publication or dataset does not currently include an investigation. Please add any experiments detailed in the publication or performed to obtain this dataset.
|
Abstract
Summary
A photochemical reduction of CO2 to CO or formic acid was shown using the bipyridine-based rhenium, ruthenium and manganese catalysts [Re(bpy)(CO)3(MeCN)][PF6], Ru(dtBubpy)(CO)2Cl2 or [Mn(dtBubpy)(CO)3(MeCN)][PF6] in combination with cyclic rhenium-based trinuclear redox photosensitizers. Turnover numbers (TONs) of up to 290 for formic acid were reached in DMA with the ruthenium complex Ru(dtBubpy)(CO)2Cl2 and photosensitizer Molecule with key KSOIVZAANOLODS-UHFFFAOYSA-T does not exist.. For CO production, TONs of up to 98 were obtained in DMF with the rhenium complex [Re(bpy)(CO)3(MeCN)][PF6] and photosensitizer Molecule with key LOLRMPNEYKEGPF-UHFFFAOYSA-T does not exist.. The experiments were conducted under visible-light irradiation (λ = 436 nm) using TEOA as sacrificial electron donor (see section SEDs below).
Advances and special progress
Re(I)-based trinuclear photosensitizers were developed and allowed for high product selectivities for CO or formic acid in CO2 reduction attempts with different bipyridine-based catalysts.
Additional remarks
Content of the published article in detail
The article contains results for the reduction of CO2 to CO or formic acid under visible-light catalysis using bipyridine-based complexes and rhenium-based trinuclear rings as photosensitizers. The catalytic system performs best in DMA for formic acid production (referring to the TON of formic acid production) and in DMF for CO production.
Catalyst
[Re(bpy)(CO)3(MeCN)][PF6]
Ru(dtBubpy)(CO)2Cl2
[Mn(dtBubpy)(CO)3(MeCN)][PF6]
Photosensitizer
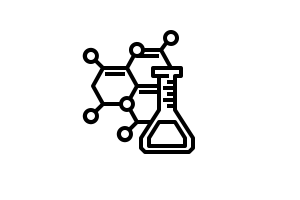
Investigation
| cat | cat conc [µM] | PS | PS conc [mM] | solvent A | . | . | λexc [nm] | . | TON CO | . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 50 | 0.05 | 436 | 27 | |||||||||
| 2. | 50 | 0.05 | 436 | 98 | |||||||||
| 3. | 50 | 0.05 | 436 | 22 | |||||||||
| 4. | 50 | 0.05 | 436 | 71 | |||||||||
| 5. | 0.05 | 436 | 6 | ||||||||||
| 6. | 0.05 | 436 | 8 | ||||||||||
| 7. | 50 | 0.05 | 436 | 20 | |||||||||
| 8. | 50 | 0.05 | 436 | 32 | |||||||||
| 9. | 50 | 0.05 | 436 | 11 | |||||||||
| 10. | 50 | 0.05 | 436 | 48 |

| cat | cat conc [µM] | PS | PS conc [mM] | e-D | e-D conc [M] | solvent A | . | . | λexc [nm] | . | TON CO | TON H2 | TON HCOOH | . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 50 | 0.05 | 436 | 20 | 72 | 290 | |||||||||||
| 2. | 50 | 0.05 | 0.03 | 436 | 16 | 49 | 280 | ||||||||||
| 3. | 50 | 0.05 | 436 | 32 | 85 | ||||||||||||
| 4. | 50 | 0.05 | 0.03 | 436 | 80 | 60 |

Sacrificial Electron Donor
In this study, the experiments were done with the sacrificial electron donor TEOA (100507).
Additives
In this study, no additives were tested.

